contents area
Public Health Weekly Report
detail content area
- Date2019-08-29 18:56
- Update2019-11-19 18:32
- DivisionDivision of Laboratory Diagnosis Management
- Tel043-719-7053
Accreditation system for national reference laboratories for infectious diseases
Park Jae-Sun, Park Ye-Eun, Kim Gab Jung, Lee Sangwon
Division of Laboratory Diagnosis Management, Center for Disease Control and Prevention, KCDC
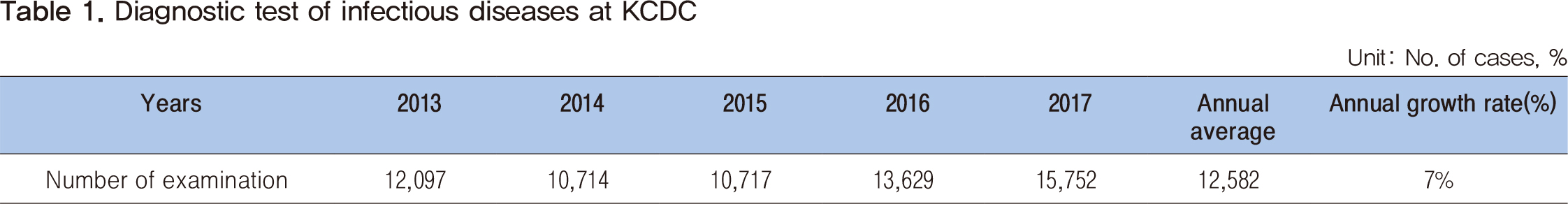
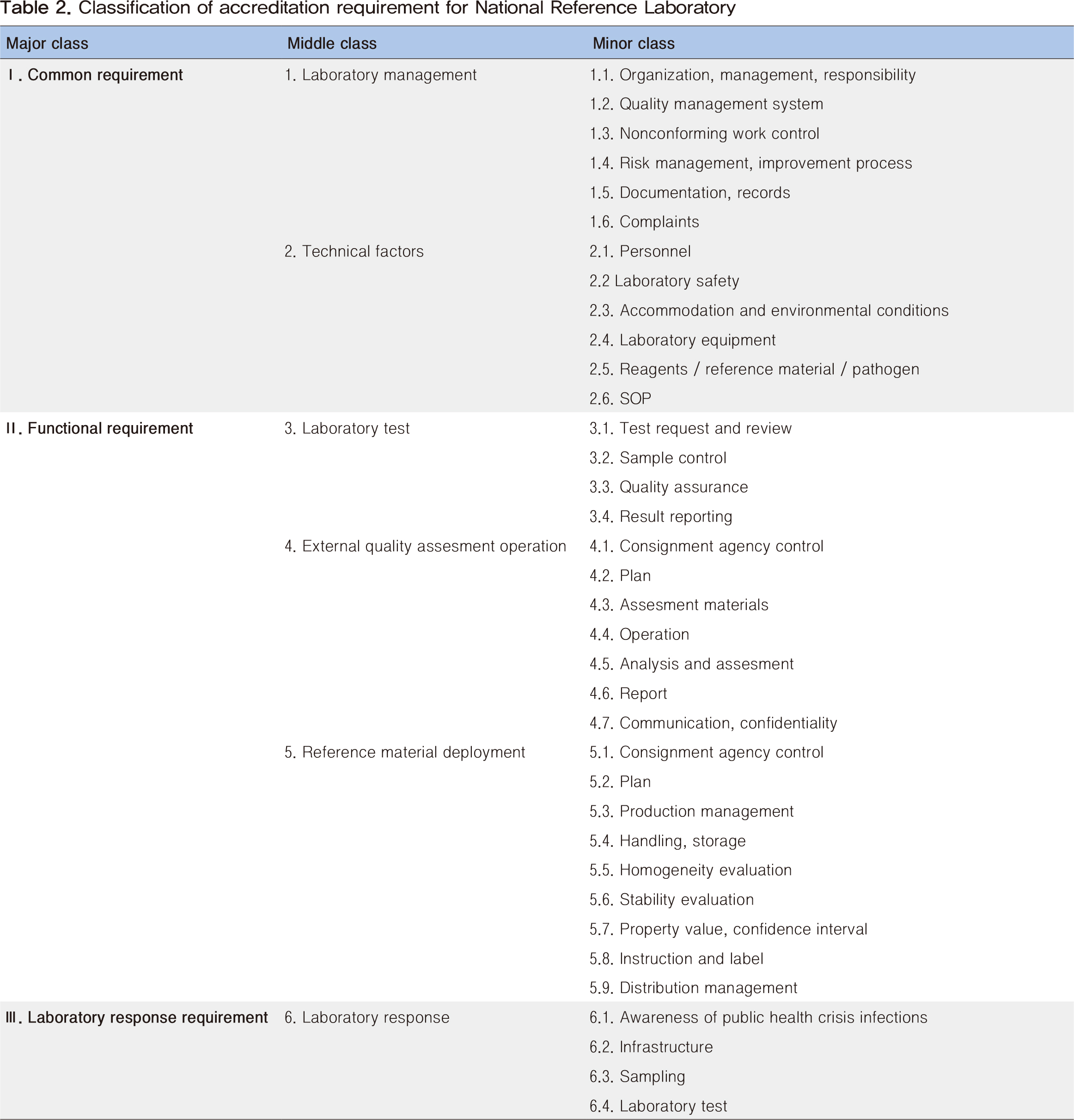
A national reference laboratory accreditation system for infectious diseases was established in 2018 to readjust the national laboratory system and set up national standards for examination systems of infectious diseases. To receive accreditation as a national reference laboratory for infectious diseases, a total of 165 requirements must be verified by a committee of experts in each field. The requirements for accreditation are categorized into the following 5 functions: management of the laboratory, examination of infectious diseases, operation of an external quality assessment program, production and distribution of reference materials, and response to an emergency. Assessment for accreditation comprises three sections, document assessment, field assessment, and comprehensive review. The system aims to accredit the laboratories for a total of 53 infectious diseases by 2023. A preliminary assessment of four laboratories was carried out, and the accreditation system was upgraded based on the results earlier this year. In addition, a laboratory assessment for a total of 14 infectious diseases is planned to be completed by the end of this year. We will be able to objectively certify and strengthen the capability of national laboratories through the accreditation system for national reference laboratories for infectious diseases. It is hoped that this effort will serve as a basis for establishing national standards for the examination of infectious diseases.
Keywords: National reference laboratory, Infectious disease, Accreditation system
 This public work may be used under the terms of the public interest source + commercial use prohibition + nonrepudiation conditions
This public work may be used under the terms of the public interest source + commercial use prohibition + nonrepudiation conditions