contents area
Chronic Infectious Disease Cohort Study
detail content area

Chronic Infectious Disease
Cohort Study
HIV/AIDS Cohort Study
Since the first establishment of HIV/AIDS cohort in 2006, we investigated the patients’ socio-demographic status, health-related lifestyle, transmission route, anxiety and depressive symptoms, family history, opportunistic infection and ART. After obtaining written informed consent from 15 hospitals nationwide, 930 HIV-infected individuals have been registered and followed up every six months. In addition, 9,351 samples have been deposited in the National Biobank of Korea, with paired data deposited. More than 30 HIV/AIDS research-related proposals were selected using cohort samples or data and proceeding sequentially. It will provide useful information for prevention of AIDS, reduction of HIV-related morbidity and mortality, and improvement of quality of life in Korean HIV-infected individuals through the HIV/AIDS cohort study.
HPV Cohort Study
The Korea HPV cohort study was established in 2009. It is an observational study of HPV-infected women aged 20-60 years. Women that are HPV-positive for both atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesions (LSIL) of the cervix were enrolled through the HPV cohort study implemented in 5 hospitals. The goals of this cohort study are as follows: (1) to study the epidemiology of persistent HPV infection, (2) to investigate whether persistent HPV infection increases the risk of low-grade and high-grade cervical lesions, and (3) to elucidate the genetic and immunological determinants of persistent HPV infection. To achieve these goals, the 685 individuals have been registered, and followed-up every 6 months. The collected samples (serum, plasma, buffy coat, Cervix cell DNA) and clinical data have been deposited at the bio-bank and database system for future HPV projects.
HCV Cohort Study
The Korea HCV cohort study was established with 5 university hospitals in 2007. This prospective and multi-center study enrolled patients with positive results for the anti-HCV antibody who completed a questionnaire survey related to aspects of diseases and risk factors focusing on HCV infection. As of 2016, 1,917 patients have been followed every 6 months. The goals of this cohort study are as follows: (1) to study the epidemiology of acute and chronic HCV infection, (2) to analyze important risk factors of HCV, and (3) to investigate the efficacy of antiviral therapy and disease outcome due to HCV infection. This study is the first nationwide HCV cohort study in our country. The data from this cohort will provide valuable information to modify optimal treatment guidelines for Korean patients infected with HCV.
HBV Cohort Study
The Korea HBV cohort study was established in collaboration with 4 university hospitals in 2015. This prospective and multi-center study enrolled patients with chronic hepatitis B who completed a questionnaire on treatment patterns and efficacy, drug resistance, long-term clinical outcomes, long-term effects after cessation of antiviral agent therapy, and safety of long-term use of such agents. 345 patients have been enrolled, and 423 blood samples have been collected. Results of this study will be used to establish practice guidelines and predict the clinical outcomes of patients with chronic hepatitis B. This cohort will provide valuable information that can be used to establish evidence-based treatment guidelines for Korean patients with chronic hepatitis B on antiviral treatments and will facilitate further research.
Workflow in the chronic infectious disease cohort study
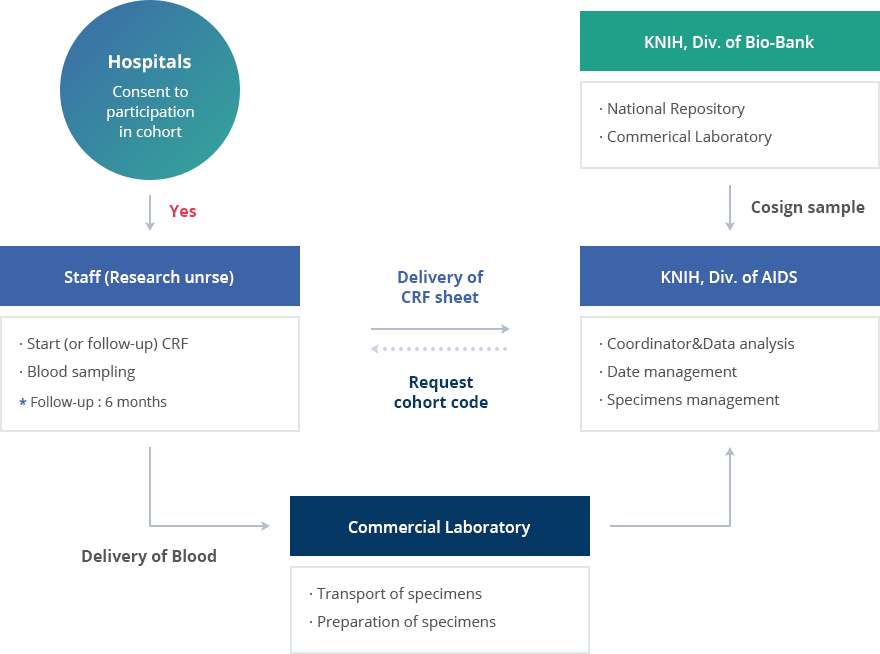
- 1. Hospitals Consent to participation in cohort
-
if yes
Staff (Research unrse)
- Start (or follow-up) CRF
- Blood sampling
* Follow-up : 6 months
-
Delivery of Blood
Commercial Laboratory
- Transport of specimens
- Preparation of specimens
-
KNIH, Div. of AIDS
- Coordinator&Data analysis
- Date management
- Specimens management
Before Cosign sample
KNIH, Div. of Bio-Bank National Repository,Commerical Laboratory
