contents area
The National Center For Stem Cell And Regenerative Medicine
detail content area

The National Center For Stem Cell And Regenerative Medicine
Stem cell research and regenerative medicine is an emerging field of medical science that focuses on the regeneration, repair, or replacement of damaged tissues and organs. The global market for regenerative medicine has been propelled by increasing demand for stem cell therapy and tissue engineering.
NCSR will help all of the stem cell researchers in the world. The missions of NCSR are to provide easily accessible stem cell resources, to share regenerative medical information, and to support clinical applications. Our GMP facility will support the production of regenerative medicine. With three independent manufacturing facilities, QC rooms and a storage room, we are able to produce 5 independent product at the same time.
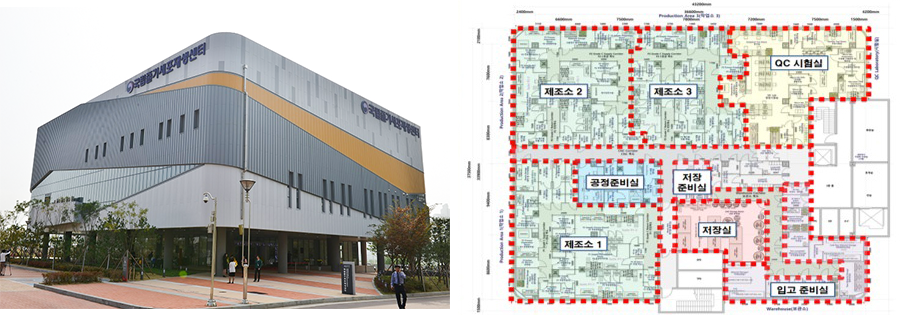
National Stem Cell Bank of Korea
The National Stem Cell Bank of Korea (NSCB) has been opened 2012 to function as part of the infrastructure for regenerative medicine research in Korea. The focus of the NSCB is on 1) pluripotent stem cell banking and distributing, 2) Quality controls of stem cells. The NSCB has prepared standard operation procedures (SOPs) to establish a standardized cell banking system for the culturing, QC, and distributing of human pluripotent stem cells.
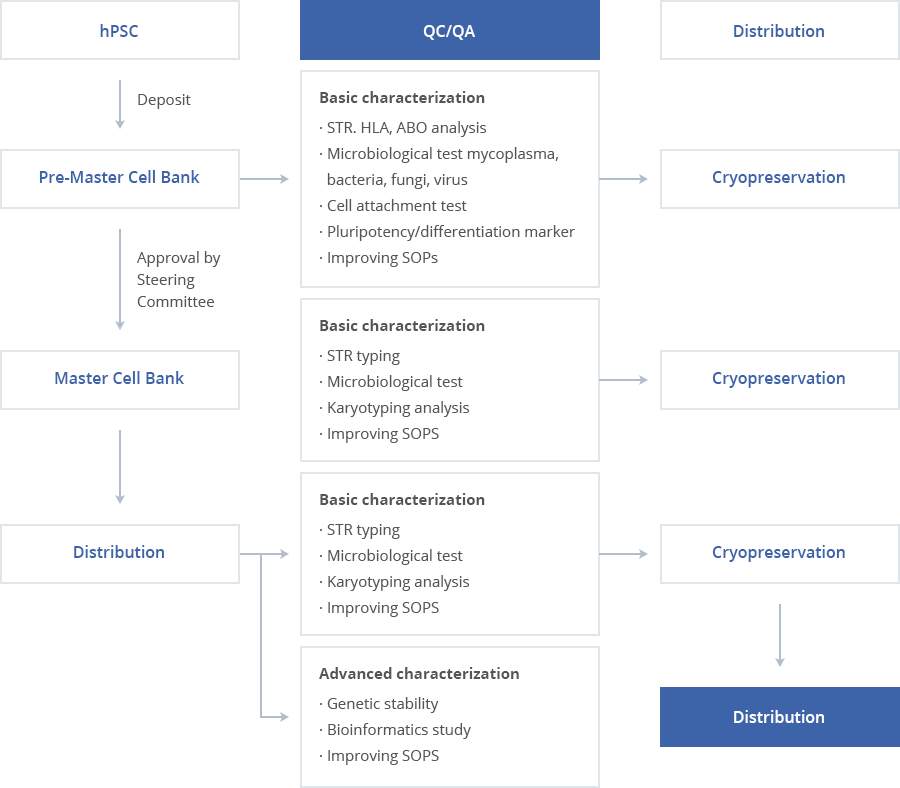
QC/QA
Basic characterization
- STR. HLA, ABO analysis
- Microbiological test mycoplasma,
- bacteria, fungi, virus
- Cell attachment test
- Pluripotency/differentiation marker
- Improving SOPs
Basic characterization
- STR typing
- Microbiological test
- Karyotyping analysis
- Improving SOPS
Basic characterization
- STR typing
- Microbiological test
- Karyotyping analysis
- Improving SOPS
Advanced characterization
- Genetic stability
- Bioinformatics study
- Improving SOPS
- hPSC
- Deposit
- Pre-Master Cell Bank
- Approval by Steering Committee
- Master Cell Bank
- Distribution
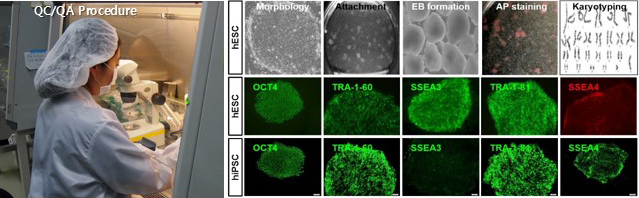
- hESC : Morpbology, Attachment, EB fomation, AP staining, Karyotyping, OCT4, TRA-1-B0, SSEA3, TRA-1-B1, SSEA4
- hiPSC:OCT4, TRA-60, SSEA3, TRA-1B4, SSEA4
Human Embryonic Stem Cell Registry
The Korea Stem Cell Registry (KSCR) was established to increase the credibility of human embryonic stem cell (hESC) lines established in Korea, and legislation became effective on January 1, 2010. The KSCR has characterized submitted lines with respect to DNA fingerprint, chromosome stability, expression of stem cell markers, and contamination with Mycoplasma; the characterization data and ethical aspects of this work, such as obtaining informed consent for the donation of surplus embryos, were reviewed by an advisory board. Information about the hESC lines is available at the KSCR website (http://nih.go.kr).
