Surveillance of Viral Infectious Disease Pathogens
Purpose of surveillance of viral infectious disease pathogens
- The goal is to detect viral outbreaks early to prevent the spread of infectious diseases. To this end, the Korea Centers for Disease Control and Prevention’s Division of Viral Diseases operates various virus surveillance systems in collaboration with local medical institutions and provincial health and environmental research institutes.
Infectious diseases subject to surveillance
- A total of 9 types of viral infectious diseases are monitored
- Class 3 infectious diseases (diseases that must be reported immediately upon occurrence): Japanese encephalitis, West Nile fever, tick-borne encephalitis
- Class 4 infectious diseases (infectious diseases monitored through sample surveillance): Norovirus, Group A rotavirus, enteric adenovirus, astrovirus, sapovirus, enterovirus (including hand, foot, and mouth disease)
Major surveillance systems
- EnterNet Korea (Waterborne and Foodborne Infectious Disease Pathogen Surveillance System): Analyzes outbreaks of acute diarrheal diseases caused by water or food.
- The target includes 5 types: Norovirus, Group A rotavirus, enteric adenovirus, astrovirus, and sapovirus, with 17 city and provincial health and environmental research institutes and 77 medical institutions participating.
- KESS (Surveillance system for enterovirus infections): Analyzes the outbreak patterns and dominant genotypes of enteroviruses that cause hand, foot, and mouth disease, aseptic meningitis, and herpetic angina in infants and young children.
- 17 city and provincial health and environmental research institutes and 83 participating medical institutions are involved.
- K VESS (Integrated Encephalitis Syndrome Pathogen Surveillance System): When suspected cases of Japanese encephalitis or tick-borne encephalitis occur, it tests for nine types of encephalitis-causing viruses to identify the cause.
- The target includes 9 types: Japanese encephalitis virus, tick-borne encephalitis virus, West Nile virus, dengue virus, Zika virus, varicella virus, adenovirus, measles virus, and enterovirus. This is a new initiative that started in 2024, currently involving 5 city and provincial health and environmental research institutes.
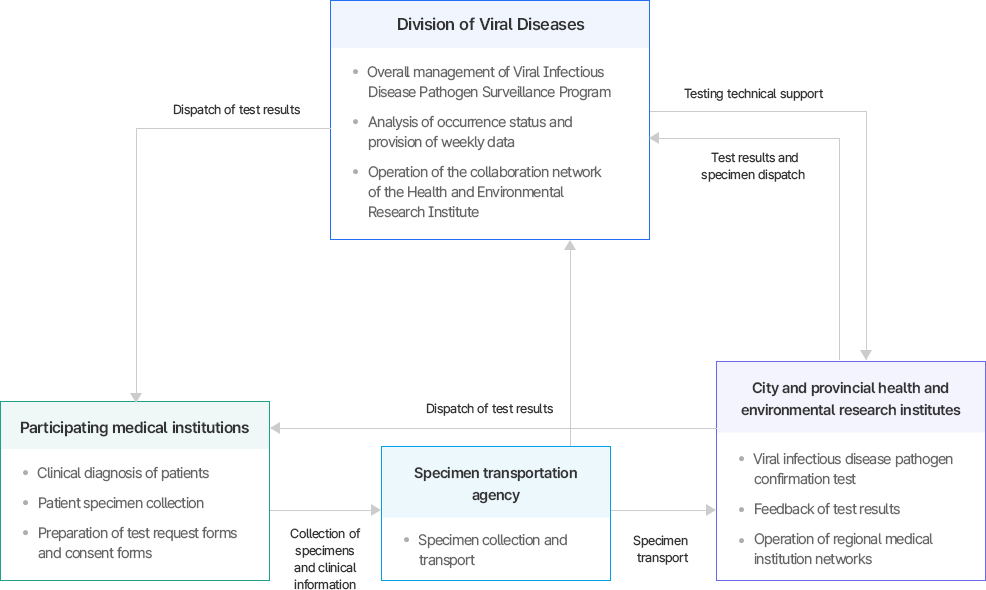
이 이미지는 바이러스성 감염병 병원체 검사가 어떻게 수행되고 결과가 공유되는지를 설명하는 체계도이다. ■ 참여의료기관 - 환자 임상 진단 및 검체 채취. - 검사 의뢰서 및 동의서 작성. - 검사 결과를 통보받음. ■ 검체 운송기관 - 참여의료기관에서 수집된 검체와 임상 정보를 수집. - 검체를 시·도 보건환경연구원 또는 바이러스분석과로 이송. ■ 시·도 보건환경연구원 - 바이러스성 감염병 병원체 확인 검사 수행. - 검사 결과를 환류하고 지역 의료기관 네트워크 운영. - 바이러스분석과에 결과를 송부. ■ 바이러스분석과 (중앙) - 바이러스성 감염병 병원체 검사사업 총괄. - 발생 현황 분석 및 주간 자료 제공. - 보건환경연구원 협력 네트워크 운영. - 검사 기술 지원 제공 및 검사 결과 취합·분석. ■ 정보 흐름 요약 1. 환자 진단 및 검체 채취: 참여의료기관에서 수행. 2. 검체 수집·이송: 검체 운송기관을 통해 연구원으로 전달. 3. 검사 수행: 시·도 보건환경연구원이 병원체 검사 및 확인. 4. 중앙 관리: 바이러스분석과가 검사사업 총괄, 결과 종합, 기술 지원 및 자료 제공. 5. 환류: 검사 결과와 발생 현황이 다시 의료기관과 연구기관으로 전달됨. ■ 종합 해석 검체와 정보는 참여의료기관 → 운송기관 → 시·도 보건환경연구원 → 바이러스분석과로 상향 보고되며, 검사 결과와 분석 자료는 다시 환류되어 현장 진단과 정책 관리에 활용된다.
Disclosure and utilization of surveillance results
- The data collected through surveillance is updated weekly on the Disease Control and Prevention Agency's website under the Infectious Disease Portal → Infectious Disease News → Publications and Newsletters → Laboratory Newsletter.
- This laboratory newsletter summarizes the latest results of each surveillance system and is used as a fundamental resource for establishing infectious disease response policies. In addition, medical institutions actively utilize this data to understand the regional epidemic situation and take appropriate treatment and prevention measures, enabling healthcare professionals to respond effectively in the field.
