Isolation, distribution, transfer of high-risk pathogens and reporting of transfer (Infectious Disease Control and Prevention Act Article 21)
- A person who has extracted a high-risk pathogen from a patient of an infectious disease, food, animal/plant, or any other environment shall, without delay, report the name of the high-risk pathogen, the name of the object from which the pathogen has been extracted, the date and time of extraction, etc. to the Commissioner of the Korea Disease Control and Prevention Agency
- A person who intends to have a high-risk pathogen distributed and transferred shall first report the name of the high-risk pathogen, its distribution and transfer plan, etc. to the Commissioner of the Korea Disease Control and Prevention Agency.
- A person who intends to have a high-risk pathogen transferred shall first report the name of the high-risk pathogen, its transfer plan, etc. to the Commissioner of the Korea Disease Control and Prevention Agency.
Reporting of high-risk pathogen extraction
- Report immediately after final confirmation of the high-risk pathogen.
 Institution extracting high-risk pathogen
Institution extracting high-risk pathogen
- Isolation report
- National management number assignment
 (Biosafety Assessment Division)
(Biosafety Assessment Division)
- Documents to submit
- High-Risk Pathogen Extraction Report Form (Schedule 7 of the Enforcement Regulations)
- High-Risk Pathogen Extraction Circumstances Report (Schedule 7-2 of the Enforcement Regulations)
- Notified of results within 10 days after receiving the report (issuance of high-risk pathogen extraction report confirmation)
High-Risk Pathogen Distribution and Transfer Report
- Before high-risk pathogen distribution and transfer
 Institution requesting to receive high-risk pathogens
Institution requesting to receive high-risk pathogens
- Distribution and Transfer Report
- Notification of distribution and transfer approval
 (Biosafety Assessment Division)
(Biosafety Assessment Division)
- Documents to submit
- High-Risk Pathogen Distribution and Transfer Report Form (Schedule 7-4 of the Enforcement Regulations)
- High-Risk Pathogen Distribution Contract or Order Form
- Usage plan for the high-risk pathogens to be received for distribution and transfer
- Permit, report confirmation, or use contract for a high-risk pathogen handling facility pursuant to Article 23 (1) of the Infectious Disease Control and Prevention Act
- Transport plan for the high-risk pathogens to be received for distribution and transfer (including information on transport route, means of transport, and transporter)
- Transfer agency contract (applicable only where an agency is handling the transfer)
- Document stating the name, education, and experience of the individuals handling high-risk pathogens
- Notified of results within 10 days after receiving the report (issuance of high-risk pathogen distribution and transfer report confirmation)
High-Risk Pathogen Transfer Report
- Before moving high-risk pathogens
 Receiving (or sending) institution for high-risk pathogens
Receiving (or sending) institution for high-risk pathogens
- Transfer report
- Notification of transfer approval
 (Biosafety Assessment Division)
(Biosafety Assessment Division)
- Documents to submit
- High-Risk Pathogen Transfer Report Form (Schedule 8 of the Enforcement Regulations)
- Usage plan for high-risk pathogens
- Transport plan for high-risk pathogens (including information on transport route, means of transport, and transporter)
- Transfer agency contract (applicable only if an agency is handling the transfer)
- Notified of results within 10 days after receiving the report (issuance of high-risk pathogen transfer report confirmation)
Permission for Introduction of High-Risk Pathogens (Article 22 of the Infectious Disease Control and Prevention Act)
- A person who intends to introduce high-risk pathogens into the Republic of Korea for the purposes of diagnosis of, academic research, etc. on infectious diseases shall obtain permission therefor from the Commissioner of the Korea Disease Control and Prevention Agency by satisfying the following requirements:
- The person shall install and operate the facilities handling high-risk pathogens referred to in Article 23 (1), or enter into a contract for the use of the same with a person who establishes and operates the facilities;
- The person shall formulate a plan for safely transporting high-risk pathogens, including emergency measures;
- The person shall designate a manager in exclusive charge of high-risk pathogens, satisfying the requirements prescribed by the Ministerial Decree of Health and Welfare.
- A person who intends to modify any of the matters permitted under paragraph (1) shall obtain permission therefor from the Commissioner of the Korea Disease Control and Prevention Agency; provided, however, that where such person intends to modify any minor matter prescribed by Presidential Decree, he or she shall report such modification to the Commissioner of the Korea Disease Control and Prevention Agency.
- Where a person who has obtained permission to introduce high-risk pathogens into the domestic environment under paragraph (1) intends to transfer the relevant high-risk pathogens after acquiring them, he or she shall designate a place to acquire them, as prescribed by Presidential Decree, and shall report, in advance, a transfer plan to the Commissioner of the Korea Disease Control and Prevention Agency pursuant to Article 21 (1).
- Where a person who has obtained permission pursuant to paragraph (1) falls under any of the following, the Commissioner of the Korea Disease Control and Prevention Agency may revoke the permission.
✓ Requirements for a dedicated manager of high-risk pathogens
- Individuals with a major and experience in the health care or biological related fields
- Individuals who have received training in handling high-risk pathogens.
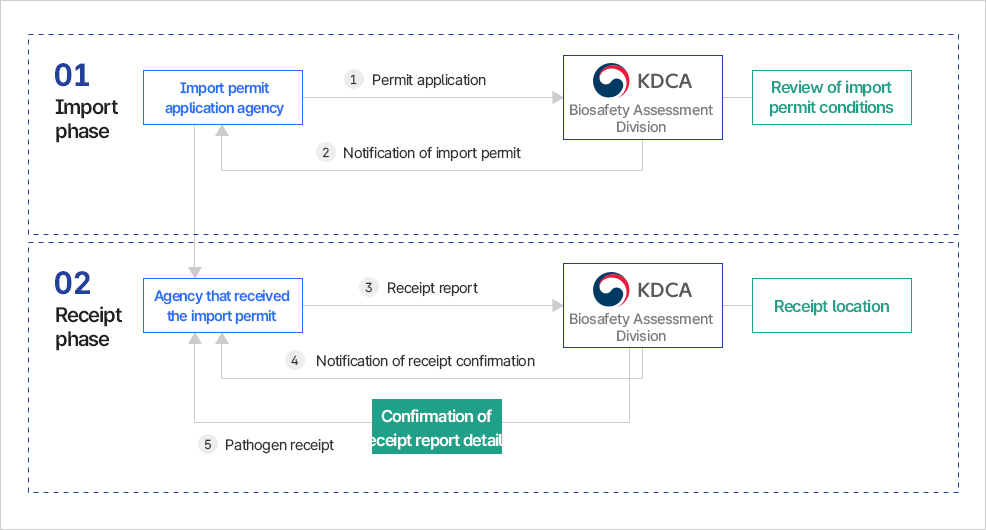
High-risk pathogen import permit
- Before bringing in (importing) high-risk pathogens (Figure, Diagram 4)
- Documents to submit
- Application Form for High-Risk Pathogen Import Permit (Schedule 9 of the Enforcement Regulations)
- Import contract (or agency contract for import if undertaken by an agent) or order form
- Usage plan for high-risk pathogens to be imported
- Transport contract or self-transport plan recording transport route, means of transport, and transporter
- Permit, confirmation of report, or usage contract for a high-risk pathogen handling facility in accordance with Article 23 (1) of the Infectious Disease Control and Prevention Act
- Document containing the name, educational background, and experience of the person handling high-risk pathogens
- Notification of results within 10 days after report receipt (issuance of high-risk pathogen import permit)
- Confirm the planned receipt date and location for high-risk pathogens to fulfill the high-risk pathogen receipt report.
✓Permission for changes to high-risk pathogen import permit and change notification
- If seeking to change items authorized in the high-risk pathogen import permit
- Documents to submit
- Application Form for Changes to High-Risk Pathogen Import Permit (Schedule 11 of the Enforcement Regulations)
- Copy of the import permit or conditional import permit
- Documents verifying the changes
High-Risk Pathogen Receipt Report
- Before receiving the high-risk pathogens after obtaining the import permit
✓High-risk pathogen receipt location
- Airports: Incheon International Airport, Gimhae International Airport, Jeju International Airport
- Ports: Incheon Port, Busan Port, Mokpo Port, Jeju Port, Gunsan Port
- Documents to submit
- Receipt Report Form for High-Risk Pathogens (Schedule 12 of the Enforcement Regulations)
- Detailed information and usage purpose for each pathogen to be received
- Where an agency performs receipt on behalf of another party: agency receipt contract, transport contract or transport plan recording transport route, means of transport, transporter, and storage information
- Notification of results within 10 days after report receipt (issuance of high-risk pathogen receipt report confirmation)
Report on the Status of High-Risk Pathogen Possession (Article 21, Paragraph 6 of the Infectious Disease Control and Prevention Act)
- A person who possesses or controls high-risk pathogens shall prepare records on the current status of possession of such high-risk pathogens on an annual basis and submit them to the Commissioner of the Korea Disease Control and Prevention Agency.
- High-Risk Pathogen Possession Status Report (Schedule 8-3 of the Enforcement Regulations)
- By January 31 of each year (as of December 31 of the previous year)
Permission for Possessing Infectious Pathogens Spread through Bioterrorism (Article 23-3 of the Infectious Disease Control and Prevention Act)
- A person intending to possess any pathogen prescribed by Decree of the Minister of Health and Welfare (hereinafter referred to as "infectious pathogens spread through bioterrorism"), among the pathogens causing infectious disease spread through bioterrorism, for the purposes of diagnosis, academic research, etc. of infectious diseases, shall obtain advance permission therefor from the Commissioner of the Korea Disease Control and Prevention Agency; provided, however, that where it is impracticable to obtain advance permission due to unavoidable causes prescribed by Presidential Decree, including cases of possessing an infectious pathogen spread through bioterrorism after extracting it from a probable patient of an infectious disease, permission shall be obtained immediately after possessing such pathogen.
- ② A person who obtains permission to introduce high-risk pathogens into the domestic environment under Article 22 (1) shall be deemed to have obtained the permission prescribed in paragraph (1).
- Any person intending to modify any of the matters permitted under paragraph (1) shall obtain permission for modification from the Commissioner of the Korea Disease Control and Prevention Agency; provided, however, that for cases of modifying any minor matter prescribed by Presidential Decree, such as replacing a person handling high-risk pathogens, a report on such modification shall be submitted to the Commissioner of the Korea Disease Control and Prevention Agency.
✓ Types of biological terrorism infectious disease pathogens(8 types)
- Bacteria: Yersinia pestis (plague bacterium), Bacillus anthracis (anthrax bacterium, excluding the Sterne strain), Clostridium botulinum (botulinum bacterium), Francisella tularensis (tularemia bacterium)
- Viruses: Ebola virus, Lassa virus, Marburg virus, Smallpox virus
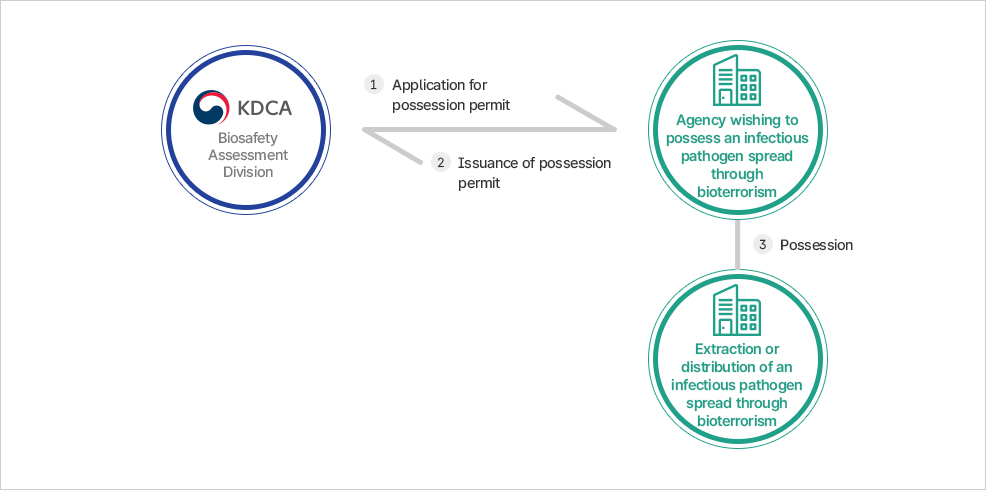
Application for permission to possess infectious disease pathogens that are potential bio-weapons
- Before possessing an infectious pathogen that is a potential bio-weapon (extraction, distribution, movement)
- Documents to submit
- Application Form for Permission to Possess Infectious Pathogens that is a Potential Bio-Weapon (Schedule 13-2 of the Enforcement Regulations)
- Plan for the Possession and Safety Management of Pathogens that is a Potential Bio-Weapon
- Permit, confirmation of report, or usage contract for a high-risk pathogen handling facility in accordance with Article 23 (1) of the Infectious Disease Control and Prevention Act
- Documents containing the name, educational background, and experience of the person handling pathogens that is a potential bio-weapon
- Notified of results within 30 days after report receipt (issuance of possession permit for infectious pathogen spread through bioterrorism)
✓ Permission for changes to permit to possess infectious pathogen that is a potential bio-weapon and change notification
- If seeking to change items authorized in the permit to possess infectious pathogen that is a potential bio-weapon
- Documents to submit
- Application Form for Changes to Permit to Possess Infectious Pathogen that is a Potential Bio-Weapon (Schedule 13-4 of the Enforcement Regulations)
- Permit to Possess Infectious Pathogen that is a Potential Bio-Weapon
- Documents verifying the changes
