Living Modified Organisms for Healthcare
- Living Modified organisms (LMO)* used for the purpose of protecting and promoting the health of the public, excluding pharmaceuticals and medical devices
- LMO and products using LMO for the production of raw materials for cosmetics Use for healthcare purposes that are not pharmaceuticals or medical devices
- LMO and products using LMO for the purpose of controlling human pathogenic microorganisms and disease vectors Use for public health purposes such as sterilizers, disinfectants, antiseptics, and pesticides
- LMO and products using LMO for the purpose of improving health and the environment, such as the prevention of environmentally related diseases Soaps, shampoos, hand washes, etc. for public/personal hygiene use
- LMO and products using LMO for surgical threads, gauze, gloves, and similar purposes Biological products and non-pharmaceutical products used for healthcare purposes
[보건의료용 유전자변형생물체의 예시]
Safety management of living modified organisms for healthcare purposes
- In Korea, according to the Transboundary Movement, Etc. of Living Modified Organisms Act (LMO Act), living modified organisms are defined by purpose, and each relevant central administrative agency implements safety management. Living modified organisms for healthcare purposes are managed by the Korea Disease Control and Prevention Agency.
- To import, produce, or utilize healthcare-related LMO, prior approval from the Korea Disease Control and Prevention Agency is required.
- Before applying for approval to import, produce, or utilize for healthcare-related LMO, a risk assessment must be conducted by the Korea Disease Control and Prevention Agency.
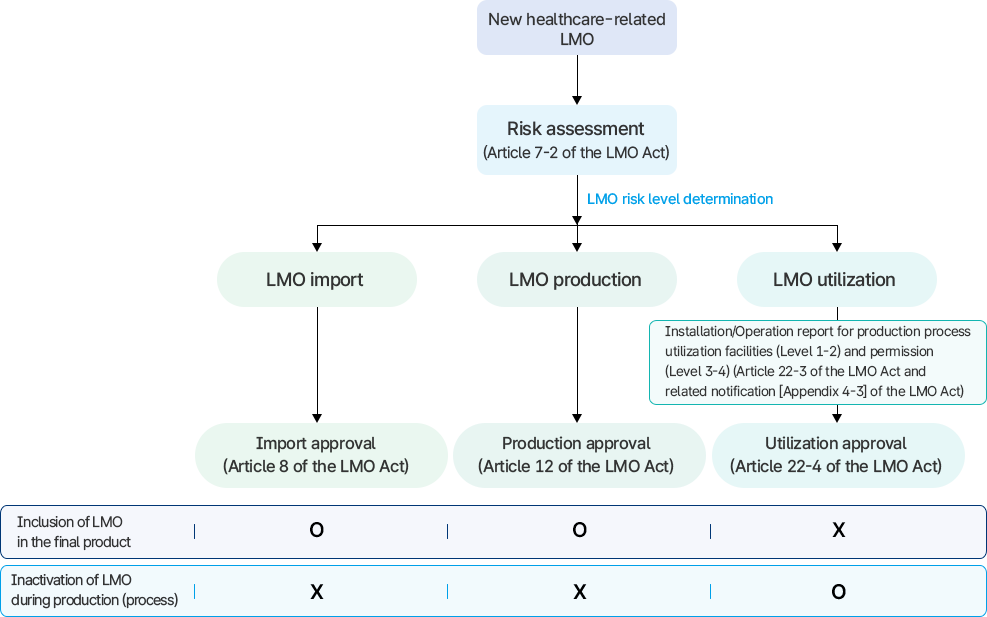
- Before applying for approval to import, produce, or utilize for healthcare-related LMO, a risk assessment must be conducted by the Korea Disease Control and Prevention Agency.
- The “production approval” and “utilization approval” for healthcare-related LMO have conceptual differences in management and operation.
- The distinction between “production approval” and “utilization approval” can be made based on whether the final product includes LMO.
- If LMO is inactivated during the production (process) and is not included in the final product, “utilization approval” must be obtained.
- If LMO is not inactivated during the production (process) and is included in the final product, “production approval” must be obtained.
Import approval for healthcare-related living modified organisms
Documents to submit
-

- Application for import approval of living modified organisms
- Risk assessment approval report issued by the Korea Disease Control and Prevention Agency
- A copy of the import contract (including import agency contract) or order form
- Transportation plan for living modified organisms
- Safety management plan for living modified organisms
- Usage plan for living modified organisms
- Government import stamp - Issuance for administrative fee (50,000 KRW)
- Prior import consent for living modified organisms intended for environmental release
* Submit only when importing LMO for environmental release.
- Safety management guide for living modified organisms
Changes to import approval details for healthcare-related LMO
| Change notification | Change approval | |
|---|---|---|
| Scope | If it falls under “minor matters” as stated in Article 8 (3) of the LMO Act, it is subject to change notification.
|
|
| Documents to Submit |
|
|
Procedure for applying for import approval of healthcare-related LMO
- Application documents: Submit the import approval application with the required documents attached.
- How to submit: Submit one printed copy of the documents either by electronic mail or by post. If submitting by email, the original must be sent by mail.
- Where to submit: Korea Disease Control and Prevention Agency, Biosafety Assessment Division
- Processing period (If there is a document supplementation period, the supplementation period is not included in the processing deadline.))
- Approval: Within 10 days
- Change notification or change approval: Within 10 days
Approval for the production of healthcare-related living modified organisms
Documents to submit
-

- Application for approval of the production of living modified organisms
- Risk assessment approval report issued by the Korea Disease Control and Prevention Agency
- Safety management plan for living modified organisms -Include information on handling and storage safety management measures, safety management personnel (handlers and management responsible persons), and status of safety management facilities (such as research facility registration confirmation and permits).
- Government import stamp - Issuance for administrative fee (50,000 KRW)
- Safety management guide for living modified organisms
Changes to approval details for healthcare-related LMO production
| Change notification | Change approval | |
|---|---|---|
| Scope | If it falls under “minor matters” as stated in Article 12 (2) of the LMO Act, a change notification is required.
|
|
| Documents to Submit |
|
|
Procedure for applying for approval of healthcare-related LMO production.
- Application documents: Submit the production approval application with the required documents attached.
- How to submit: Submit one printed copy of the documents either by electronic mail or by post. If submitting by email, the original must be sent by mail.
- Where to submit: Korea Disease Control and Prevention Agency, Biosafety Assessment Division
- Processing period (If there is a document supplementation period, the supplementation period is not included in the processing deadline)
- Approval: Within 10 days
- Change notification or change approval: Within 10 days
Approval for the use of healthcare-related living modified organisms
Documents to submit
-
- Application for approval of the use of living modified organisms
- Risk assessment approval report issued by the Korea Disease Control and Prevention Agency
- Safety management measures regarding handling, storage, etc
- Status of specialized personnel and facilities necessary for safety management
- Government import stamp -Issuance for administrative fee (50,000 KRW)
- Safety management guide for living modified organisms
Changes to approval details for healthcare-related LMO use
| Change notification | Change approval | |
|---|---|---|
| Scope | If it falls under “minor matters” as stated in Article 22-4 (2) of the LMO Act, a change notification is required.
|
|
| Documents to Submit |
|
|
Procedure for applying for approval of healthcare-related LMO use
- Application documents: Submit the application for approval of use with the required documents attached.
- How to submit: Submit one printed copy of the documents either by electronic mail or by post. If submitting by email, the original must be sent by mail.
- Where to submit: Korea Disease Control and Prevention Agency, Biosafety Assessment Division
- Processing period (If there is a document supplementation period, the supplementation period is not included in the processing deadline.)
- Approval: Within 10 days
- Change notification or change approval: Within 10 days
