Standardization of Diagnostic Tests for Chronic Diseases
Purpose
- Inaccurate diagnostic tests can cause problems such as loss of treatment opportunities, increased national medical costs, and reduced reliability of national statistical indicators.
- Establishment and operation of a standardization system to improve the accuracy of major diagnostic tests for chronic diseases Diabetes (glycated hemoglobin, glucose), kidney disease (creatinine), dyslipidemia (total cholesterol, triglycerides, HDL [high-density lipoprotein] & LDL [low-density lipoprotein] cholesterol)
Implementation System
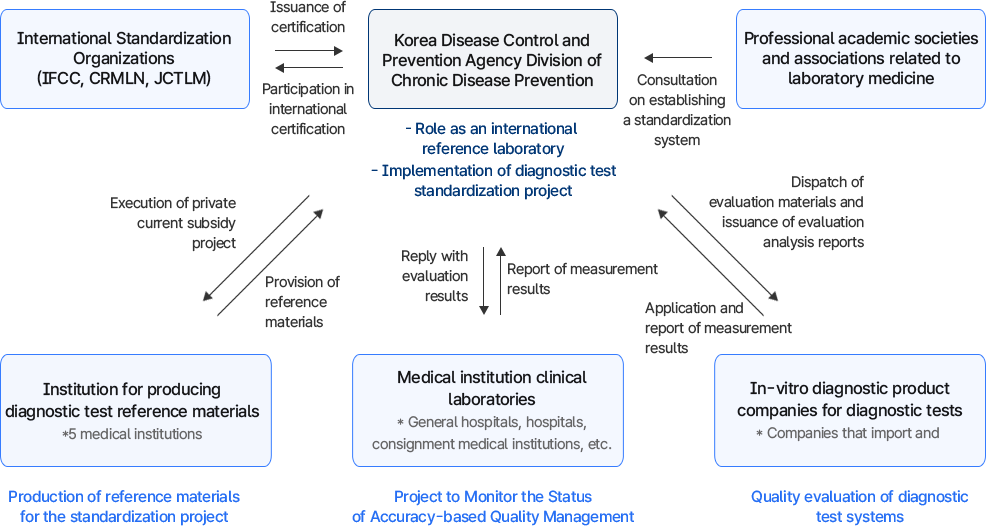
이 이미지는 진단검사 표준화 사업의 추진체계를 나타낸 도식이다.
- 국제 표준화 기구 (IFCC, CRMLN, JCTLM): 인증서 발급, 국제 인증 참여.
- 질병관리청 만성질환예방과: 진단검사실 역할 표준화 사업 수행, 표준화 체계 구축 지원.
- 진단검사의학 관련 전문 학·협회: 표준화 관련 자문 제공.
- 진단검사 표준물질 생산기관 (5개 의료기관): 표준화 사업용 표준물질 생산.
- 의료기관 진단검사실 (종합병원, 대학병원, 수탁의료기관 등): 측정 결과 보고, 정핵도 기반 질 관리 모니터링 사업 참여.
- 진단검사 시약·장비 수입 및 제조 회사: 진단검사시스템 품질 평가 수행.
- 운영 방식: 측정 결과 보고 → 평가물질 발송 및 분석 → 평가 결과 회신 → 인증 발급.
의미: 국제 기구, 정부, 학·협회, 의료기관, 기업이 협력하여 진단검사 시스템의 품질과 정확도를 보장하고, 표준화 기반의 신뢰성 있는 검사체계를 마련하는 과정을 보여준다.
Project Details
▲Operation of a national reference laboratory, ▲production of diagnostic test reference materials, and ▲implementation of diagnostic test standardization projects for the standardization of diagnostic tests.
- (Operation of a national reference laboratory) Operation of a national reference laboratory for laboratory medicine that meets international standardization requirements, including establishing standard testing methods for diagnostic test items and obtaining international certificati
- (Production of diagnostic test reference materials) Establishment of a system for producing the reference materials needed for the diagnostic test standardization project Consignment production through a diagnostic testing institution capable of large-scale blood supply and verification of reference materials
- (Diagnostic test standardization project)
| Project Name | Details | Target | Schedule |
|---|---|---|---|
| Project to Monitor the Status of Quality Management in Diagnostic Testing Institutions | Evaluation of the accuracy of diagnostic testing institutions, and preparation of the current status of quality management and basic data | Diagnostic testing institutions | Twice a year |
| Project for Quality Evaluation of Diagnostic Test Systems | Supply of high-quality in-vitro diagnostic products by analyzing accuracy, precision, total error, linearity, etc. | In-vitro diagnostic product companies | Twice a year |
| Measurement of reference values for reference materials | Measurement of reference values for reference materials used in diagnostic test quality management | Reference materials used for quality control | Always |
